That left the study in Guinea, where Ebola is still infecting new victims, as the only real hope for demonstrating the efficacy of a vaccine. At the time, though, officials did warn that outbreaks in Guinea and Sierra Leone ran the risk of bringing the virus back to Liberia, where more than 4,000 people died after contracting it.
However, it’s believed that this number will fall as it’s administered to more people.
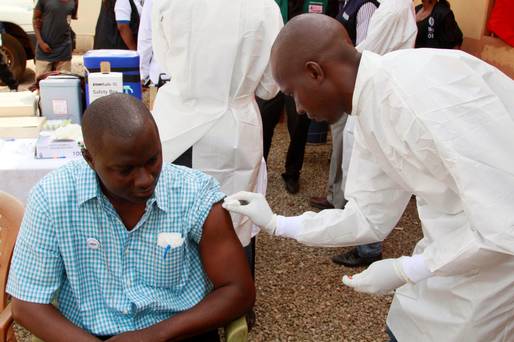
The deadly Ebola virus is getting closer to having a vaccination. They can affect many organs, damage blood vessels and affect the body’s ability to fight infection.
The vaccine is known as VSV-EBOC.
Titled “Ebola, Ca Suffit” (Eboloa, that’s enough), the trial, which began on March 23, was set up by a major global cooperation involving the WHO and experts from Norway, France, Switzerland, USA, UK and Guinea.
After it became clear that vaccinating people immediately was the best strategy, the worldwide team of researchers stopped waiting to vaccinate those at high-risk of infection.
“This is an extremely promising development”, said Margaret Chan, director-general of the World Health Organization, in a statement.
“This (vaccine) could be the key that we’ve been missing to end the outbreak”, Mr Neuman said.
Those involved in funding the trial called it a “remarkable” result and said the co-operation between organisations and countries that allowed it to come about augured well for future outbreaks of risky viruses. The other was the changing nature of the Ebola epidemic.
Only the immediately vaccinated people were 100 per cent Ebola-free.
The Canadian vaccine is the first experimental Ebola vaccine to protect against the disease.
A second study group of 3,528 people were injected with the vaccine three weeks after they’d potentially been exposed to Ebola. Only 16 of the people who were not immediately vaccinated were diagnosed with the disease after ten days.
Testing of the vaccine will also be extended to medical workers who have been working with affected patients to protect them from the disease.
Test results will now go to government regulatory agencies. It could still be awhile before the vaccine is licensed and made widely available. Such a permit would enable production of the vaccine for future Ebola epidemics.
However, there are just four documented cases left in Guinea and only three in Sierra Leone.
Another major trial in Liberia, which had aimed to recruit some 28,000 subjects, had to stop enrolling after only reaching its mid-stage target of 1,500 participants. “It is a single-dose vaccine, and the results are more than I could possibly have expected”.
SENTINEL REPUBLIC



